
Chemistry Obj
01-10 CDCCDACEBB
11-20 DACADDAAAD
21-30 CCACDBCDCA
31-40 BECDDCACEE
41-50 BDAACDBCAE
51-60 CBDCDCAABA
Completed ✅
(2a)
(i) The periodic law states that the physical and chemical properties of elements are periodic functions of their atomic numbers.
(ii) I. There are 12 neutrons present in the nucleus of the element X.
II. It belongs to Group 17 (halogens).
III. The atomic number of the element is 17.
IV. It has 7 valence electrons.
(b)
(i) Differences between cracking and reforming:
– Cracking breaks down large hydrocarbon molecules into smaller ones, while reforming converts low-quality gasoline into high-octane gasoline.
– Cracking is a thermal decomposition process, while reforming is a catalytic process.
(ii) Number of moles of ethane = 0.200/8 = 0.025
Volume of ethane at s.t.p. = 0.025 * 22.4 = 0.56 dm^3
NUMBER 4

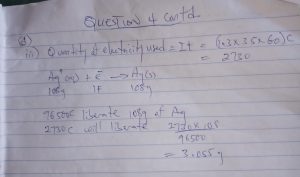
NUMBER 5



Leave a Reply