
2023 chemistry
(1ai)
(CHOOSE ANY BEST 3)
(i) Sulphur is used to produce sulphur(IV)oxide for manufacturing tetraoxosulphate(VI)acid
(ii) Sulphur is used in the vulcanization of rubber.
(iii) Sulphur and some of its products are used as fungicides and insecticides for spraying crops.
(iv) Sulphur is used to manufacture the bleaching agent used in the pulp and paper industry.
(1aii)
(CHOOSE ANY BEST 2)
(i) Hydrogen Sulphide is a colorless gas with a repulsive smell like that of a rotten egg.
(ii) It is very poisonous.
(iii) It is about 1.18 times denser than air.
(iv) It burns with a pale blue flame.
(1aiii)
Soaps are made from natural ingredients, typically fats or oils, and an alkali (such as sodium hydroxide or potassium hydroxide) in a process called saponification. WIHILE Detergents are synthetic (man-made) cleaning agents composed of various chemicals, including surfactants.
OR
Soaps are effective at removing dirt, grease, and oil, but they may not perform as well in hard water conditions. WHILE Detergents are generally more effective than soaps in all water conditions, including hard water, due to their ability to work with the mineral ions present.
(1aiv)
(CHOOSE ANY BEST 1)
(i) Detergents are used in various personal care products, such as shampoos, body washes, and hand soaps.
(ii) Detergents are specifically designed for cleaning dishes, cutlery, glasses, and cookware.
(iii) Detergents are used for general household cleaning tasks.
(1bi)
(1bii)
(1biii)
(1c)
(3)
(i) Classifying the alkanols:
– Butan-2-ol: secondary alkanol
– 2-methylpropanol: secondary alkanol
– 2-methylpropan-2-ol: tertiary alkanol
(ii) Two methods to prepare ethanol commercially:
– Fermentation: Ethanol can be produced by the fermentation of sugars using yeast. This is commonly used to produce alcoholic beverages and biofuels.
– Hydration of ethene: Ethanol can be produced by the hydration of ethene (ethylene) in the presence of a catalyst, such as phosphoric acid.
(iii) To calculate the relative molecular mass of Z, we need to compare the rates of effusion or diffusion of Z and hydrogen. The rate of effusion/diffusion is inversely proportional to the square root of the molar mass. Since hydrogen diffuses 6 times as fast, the molar mass of Z is 6 times larger than the molar mass of hydrogen. Therefore, the relative molecular mass of Z is 6 * 2 = 12.
(bi) The electronic configuration of oxygen using s, p, d, f notation is 1s2 2s2 2p4.
(ii) Three physical properties of oxygen:
(i) Oxygen is a colorless and odorless gas.
(ii) It is slightly soluble in water.
(iii) Oxygen supports combustion and is necessary for respiration.
(iii) Two rules for naming alkenes:
1. Alkenes must have the ending “-ene” in their name.
2. The longest continuous carbon chain containing the double bond is used as the base name, and the position of the double bond is indicated by the lowest possible number.
(iv) Equation for the oxidation reaction of ethene:
Ethene + Oxygen → Carbon Dioxide + Water
C2H4 + O2 → CO2 + H2O
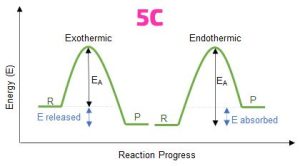
(ci) Endothermic reaction: A reaction that absorbs heat (energy) from the surroundings, resulting in a decrease in temperature.
(ii) Equation for the laboratory preparation of hydrogen using dilute tetraoxosulphate(VI) acid and Zinc:
Zn + H2SO4 → ZnSO4 + H2
(iii) The type of reaction involved in (cii) is a displacement reaction, where zinc displaces hydrogen from the acid to form hydrogen gas.
(iv) Three uses of hydrogen:
(i) Hydrogen is used as a fuel for combustion engines and fuel cells.
(ii) It is used in the production of ammonia for fertilizer and other chemicals.
(iii) Hydrogen is used in the hydrogenation of oils and fats in the food industry.
(5ai)
a base is a substance that can accept protons or donate pairs of electrons. Bases typically have a pH greater than 7 and can neutralize acids.
(ii) Examples of basic oxides include calcium oxide (CaO) and sodium oxide (Na2O).
(iii) Two uses of iodine are:
(i)Iodine is used as an antiseptic to disinfect wounds and prevent infections.
(ii) Iodine is also used in the production of X-ray contrast agents, which helps in visualizing organs and tissues during medical imaging.
(iv) One difference between an aliphatic and aromatic hydrocarbon is the structure. Aliphatic hydrocarbons are linear or branched chains of carbon and hydrogen atoms, while aromatic hydrocarbons contain a ring structure with alternating double bonds.
(v)To calculate the vapour density of XCl3, we need to know the molar mass of X. Without additional information on the element X, we cannot calculate the vapour density.
(5bi)Three factors that affect the rate of a chemical reaction are:
(i)Concentration: Increasing the concentration of reactants generally increases the rate of reaction.
(ii)Temperature: Higher temperatures usually increase the rate of reaction as particles have more energy and move faster.
(iii) Catalysts: Catalysts are substances that can speed up a reaction by providing an alternative reaction pathway with a lower activation energy.
(ii)The law of conservation of energy states that energy cannot be created or destroyed in an isolated system; it can only be transferred or transformed from one form to another.
(iii) Two examples of chemical change are:
(i)Combustion, where a substance reacts with oxygen and releases heat and light.
(ii)Rusting of iron, where iron reacts with oxygen in the presence of water and forms iron oxide.
(5c) diagram below
more solutions loading

Leave a Reply